Vertebral axial decompression therapy for pain associated with herniated or degenerated discs or fac
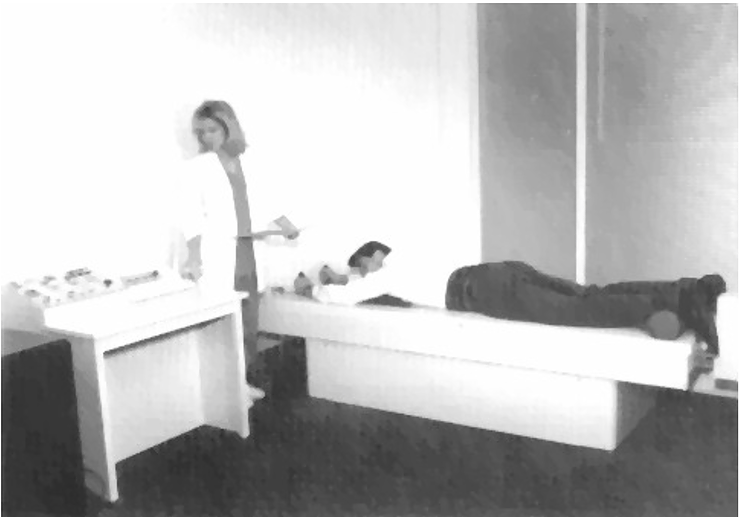
Figure 1: Patient undergoing treatment on the VAX-D Therapy Table
This article is reprinted with the permission of the authors from the Journal of Neurological Research, Volume 20.
Earl E. Gose, William K. Naguszewski* and Robert K. Naguszewski*
Department of Bioengineering, University of Iliinois at Chicago, Chicago, IL. USA
*Coosa Medical Group, Rome, Georgia, USA
The outcomes of vertebral axial decompression (VAX-D) therapy for patients with low back pain from various causes are reported. Data was collected from twenty-two medical centers for patients who received VAX-D therapy for low back pain, which was sometimes accompanied by referred leg pain. Only patients who received at least ten sessions and had a diagnosis of herniated disc, degenerative disc, or facet syndrome, which were confirmed by diagnostic imaging, were included in this study; a total of 778 cases. The average time between the initial onset of symptoms and the beginning of this therapy was 40 months, and it was four months or more in 83% of the cases. The data contained the patients' quantitative assessments of their own pain, mobility, and ability to carry out the usual 'activities of daily living'. The treatment was successful in 71% of the 778 cases, when success was defined as a reduction in pain to 0 or 1, on a 0 to 5 scale. Improvements in mobility and activities of daily living correlated strongly with pain reduction. The causes of back pain and their relationship to this therapy are also discussed. [Neurol Res 1998; 20: 186-190].
INTRODUCTION
For most patients, the cause or causes of persistent low back pain remains poorly understood. Although imaging procedures, including CT and MRI, are able to accurately define structural pathology, the correlation of these anatomic findings with physiology, back pain, and other clinical complaints is imprecise1. Although surgical decompression, epidural blocks, and spinal instrumentation can sometimes help patients suffering from back pain, these treatments do not completely take the biomechanical function of the disc into account, and may leave patients unrelieved of their suffering. In addressing the dysfunction of the disc with discectomy or surgical instrumentation, the biomechanical and physiological function of the disc is permanently disrupted.
Mechanical low back pain is usually aggravated by activities that increase axial loading on the spine, such as sitting, standing, and lifting. Patients may describe some relief with walking, but more particularly, by lying down, which unloads the spine and reduces intradiscal pressure (2,3). The causes of mechanical low back pain may include degenerative disc disease, degenerative spondylosis with limitation of range of motion, facet arthropathy, relative lateral recess stenosis from a combination of the above, microenvironment presure changes affecting the thecal and epidural space from disc bulging, subligamentous and/or extruded herniation, and segmental instability.
Pain generation from degenerative disc disease is probably multifactorial. A number of potential mechanisms are specifically addressed by the lumbar vertebral body separation achieved during therapy. With aging, disc desicction occurs, disc height is lost, and this process is accelerated with activities which produce high physical loading of the lumbar spine (4). Osteophytes develop along the anterolateral and posterior border of the vertebral bodies, and facet arthropathy increases as degenerative disc change advances (5). Normal vertebral body separation is lost as the disc degenerates. Redundancy of the posterior longitudinal ligament and ligamentum flavum combine with osteophyte encroachment upon the neuroforamen or central canal, resulting in stenosis at these sites, which is increased by axial loading of the spine.
The blood supply to the nerve roots of the cauda equina is sensitive to compression. Even at pressures of only 5-10 mmHg, the flow in over 20% of the venules was completely stopped (6). Flow in all the capillaries stopped at pressures between 20 and 50 mmHg. A pressure of 30 mmHg is slightly less than one pound per square inch, so solute transport is easily reduced. Even vertebral distractions (increased separation) of 1 or 2 mm per disc would reduce ligamental redundancy and help to restore canal/foraminal patency, reduce venous congestion and increase axoplasmic flow. Furthermore, the effects of lumbar spine lengthening may be sustained for a period of time after lumbar distraction has been stopped.
Twomey (7) placed lumbar vertebral columns removed from 23 male cadavers under 9 Kg of sustained traction for 30 min and measured an average increase in length of 9 mm. Thirty minutes after traction was removed, 13 of the 23 specimens had returned to baseline length, but the remaining 10 spines showed residual elongations ranging from 0.3 mm to 4 mm. Additionally, the data suggested that sustained traction had had a longer lasting effect on elderly spines. The mechanism of this residual deformation was not elaborated upon by the author, but disc rehydration may have been a factor since each column was soaked in normal saline and remained saturated by periodic additions of saline to a close fitting bag surrounding each column during the study.
That lumbar traction, if adequately applied, can effect physical change in patients suffering from back pain is well described by Gupta and Ramarao (8). They used water soluble contrast medium and epidurography to study 14 patients with prolapsed intervertebral disc syndrome before and after 10 to 15 days of continuous traction. Ten patients showed definite clinical improvement, with reduction in back pain and sciatica. Nine of these patients showed complete resolution of the defect on epidurogram and one of them showed partial reduction. The authors concluded that disc protrusion may be safely treated by traction. Mathews also demonstrated the effectiveness of lumbar traction in two patients by epidurography. Disc protrusions were decreased and an average vertebral distraction of 2 mm per disc space was shown in radiography (9). Judovich found that a traction force of approximately 26% of the body weight was needed just to overcome the resistance between the lower half of the patient and a (nonsplit) table (10).
Intuitively, lumbar traction should be successful in alleviating many of the conditions which cause low back pain and associated radiculopathy. Unfortunately, studies of clinical efficacy have yielded equivocal results. Previously, the successful application of lumbar traction has been limited by patient tolerance and the design of mechanical devices. Patients had difficulty tolerating the forces needed to relieve pain if delivered continuously. Furthermore, the thoracic corsets worn by patients to prevent movement on the table were uncomfortable, restrictred respiration, and can compromise venous return to the heart. Technological advances have now led to the development of equipment that has been found to achieve decompression of lumbar discs without stimulating the reactive reflexes of the lumbar musculature that can otherwise overcome efforts to effectively distract vertebral bodies.
The VAX-D therapy table is shown in Figure 1. The split table design eliminates frictional resistance between the patient and the table and allows controllable effective axial distraction tensions to be applied to the lumbar vertebral column. The equipment applies distractive forces in a gradual, progressive fashion, designed to achieve distraction of the vertebral bodies without eliciting reactive reflex muscular resistance. A portion of a typical chart recording of the tensile force applied to a patient's spine as a function of time is shown in Figure 2. Each decompression phase, during which the tension is increased, normally lasts for one minute. The force is increased more slowly in the latter part of the decompression phase. The tension is then gradually decreased, over a period of 30 sec, to about 20 pounds, which is maintained during the rest phase. Another cycle then starts. The avoidance of paravertebral muscle contraction, stimulated by homeostatic proprioceptor and axon reflex mechanisms allows the distraction of the vertebral bodies necessary to achieve decompression of the intervertebral disc. The therapy is administered via an automated logic control mechanism which systematically applies distractive tensions and rest periods in a cyclic fashion. The typical therapy session consists of 15 cycles of tension and relaxation. This periodic process allows patients to withstand stronger forces than can be tolerated when static techniques are used and it promotes accommodation and relaxation during the therapy session. The upper body is fixed by means of the patient grasping adjustable hand grips, designed to eliminate the use of a thoracic corset. Consequently, there is no risk of circulatory or respiratory compromise. The pelvis is secured with a specially designed harness that adjusts snugly and applies forces primarily to the lateral pelvic alae, thus minimizing anterior-posterior pressures and reactive muscle spasm during the distractive period of each cycle.
VAX-D treatment has been shown (11) to decompress the nucleus pulposus to pressures below - 100 mmHg. This creates a tremendous potential diffusion gradient across the disc space, which is otherwise an avascular structure. Glucose and oxygen enter the disc at the end plate region while sulphate ions needed for the production of new glycosaminoglycans enter from the annulus fibrosis (12). Thus therapy may augment nutrient flow into the disc, facilitating structural restoration of the disc and promoting disc rehydration, since proteoglycans bind water (13). These effects may be cumulative with repetitive therapy sessions.
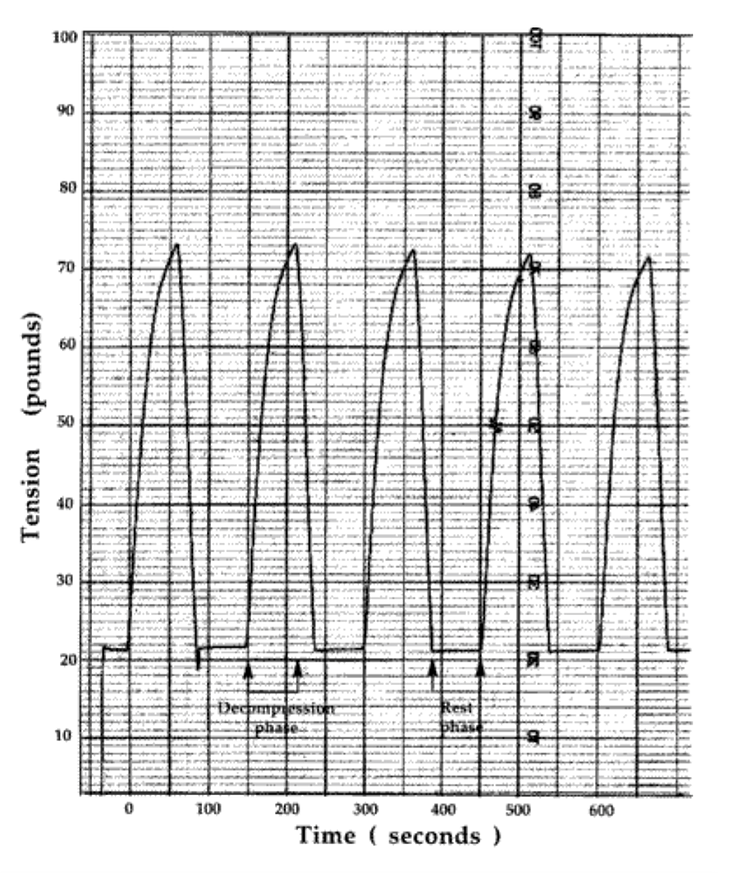
Figure 2: chart recording of tension versus time for five cycles of the typical 15-cycle VAX-D Therapy session
MATERIALS AND METHODS
Data was collected from twenty two medical centers in the USA for patients who received VAX-D therapy for low back pain. Only patients who received at least 10 treatments and had a diagnosis of herniated disc, degenerated disc, or facet syndrome, which was confirmed by imaging studies, were included in the study. The average number of treatments was 17 for facet syndrome, 19 for degenerative disc disease, and 20 for other diagnoses. The data contained the patients' assessment of their own pain, mobility, and ability to walk and sit. The pain scale ran from no pain (0) to severe pain (3). The mobility limitation scale was: No limitation (0), slightly limited (1), very limited (2), and completely immobile (3). The activity limitation scale was: walks frequently (0), walks occasionally (1), chairfast (2), and bedfast (3(). The treatment schedule, including the use of other modalities, the duration and frequency of VAX-D therapy, and medication was also recorded, as well as the patient's history. The symptoms were recorded at the beginning, mid-point, and end of the treatment schedule. The patients' satisfaction with the treatment was quantified as: not satisfied (0), slightly satisfied (1), very satisfied (2), and completely satisfied (3).
The data were divided into five groups:
The first group which contained 34 cases, included all patients with extruded herniated discs, whether or not additional lesser problems were present.
The second group contained 195 cases of multiple herniated discs, without extrusion, with or without degenerative disc disease.
The third group consisted of 382 patients with a single herniated disc, regardless of degenerative disease.
The fourth group contained 147 cases of degenerative disc disease, without herniation.
The fifth group contained 19 cases with facet syndrome. Five cases of facet syndrome which had a pain reduction to 0 or 1 before 10 treatments, and one that had a reduction to 2, received less than 10 total reatments, so they were not included in the data base.
RESULTS
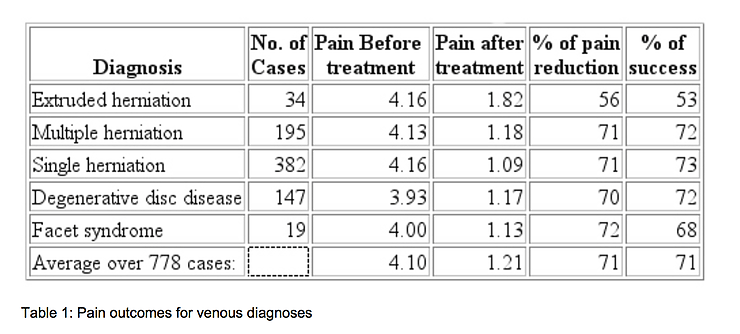
If treatment success is defined as a reduction in pain to 0 or 1 on a 0 to 5 scale, the treatment was successful in 71% of the 778 cases. The success rate varied from 53% for the patients with extruded herniated discs, to 73% for patients with a single herniated disc. It was 72% for people with multiple herniated discs and 68% for facet syndrome. On a pain scale of 0 to 5, the people with extruded herniated discs had an average pain of 4.16 at the beginning of treatment and an average of 1.82 after treatment, a reduction of 56%. The cases of multiple herniated discs went from 4.13 to 1.18, a reduction of 71%. The patients with a single herniation had a reduction from 4.16 to 1.09, or 71%. The degenerative fisc cases reduced from 3.93 to 1.17, a 70% reduction. The patients with facet syndrome had a reduction of 4.00 to 1.13, a 72% reduction in pain. Overall, 71% of the patients experienced a reduction in pain to 0 or 1. The reduction in the average pain score was also 71%. One percent of the patients reported increased pain, 7% had no change, 92% improved by 1 unit or more, 87% improved by 2 units or more, and 70% improved by 3 units or more. A summary of these findings is shown in Table 1.
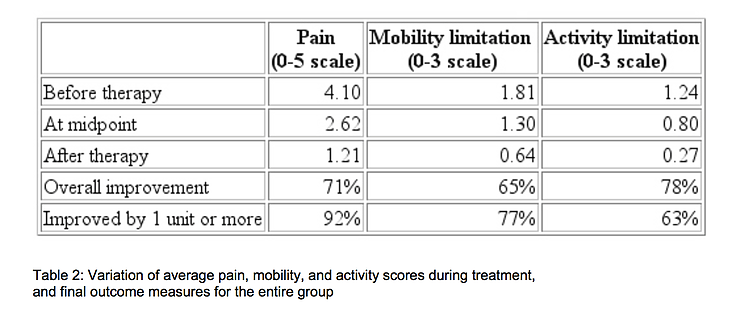
Table 2 shows how the average pain, mobility, and activity scores for the entire group of 778 patients improved during treatment. Although 51% of the pain reduction occurred during the first half of the course of treatment, 56% of the mobility improvement and 55% of the activity improvement occurred during the last half.
On a rating scale of 0 to 3, increases in spine mobility of one grade or more was seen in 77% of the patients with mobility limitations. Functional increases of 1 or more grades in the activity score was recorded in 78% of the patients who, before treatment were either unable to walk or capable of only limited walking. The coefficient of linear correlation14 between mobility and pain scores was 0.72. Between pain and activity the correlation was 0.60, and between activity and mobility it was 0.59. On a scale of 0 to 3, the average satisfaction with treatment was 2.4, which lies between 'very satisfied' and 'completely satisfied'.
In this study, 31 patients had previous lumbar disc surgery. MRI scans showed scar tissue that could potentially entrap nerve roots. Despite this, 84% of this group's pain scores and 71% of their mobility scores and 61% of their activity scores improved by one unit or more with therapy, and 65% of their pain scores were reduced to 0 or 1. Vertebral axial decompression was well tolerated.
Vertebral axial decompression therapy outcomes: Earl E. Gose et al.
DISCUSSION
We consider VAX-D therapy to be a primary treatment modality for low back pain associated with lumbar disc herniation at single or multiple levels, degenerative disc disease, facet arthropathy, and decreased spine mobility. Physiology (pain and mobility) and pathology correlate imprecisely. We believe that post-surgical patients with persistent pain or "Failed Back Syndrome' should not be considered candidates for further surgery until a reasonable trial of vertebral axial decompression has been tried.
Low back mobility increased subsequent to therapy and correlated well with pain reduction. Both of these factors are important in areas such as Workers Compensation and personal injury. Estimates of permanent partial impairment rely heavily on mobility aspects, as seen in the AMA Guides to the Evaluation of Permanent Impairment, 4th edition. Although allowance for pain is made in the percentage of impairment, the determination of impairment is made by determination of spine mobility using the range of motion model. By definition no patient can be assigned any impairment rating until maximum medical improvement (MMI) is reached. We submit that patients can usually be brought to a higher level of MMI by this therapy because of the anticipated improvements in mobility.
In summary, the pain, activity, and mobility scores were all greatly improved after therapy. VAX-D by its unique design may more precisely address the physiology of persistent low back pain than other conventional therapies.
We consider it to be a front line treatment for degenerative spondylosis, facet syndrome, disc disease and nonsurgical lumbar radiculopathy.
REFERENCES
Heldeman S. North America Spine Society: Failure of the pathology model to predict low back pain. Spine 1990; 15:718-724.
Wheeler A.D. Diagnosis and management of low back pain and sciatica. Am Family Physician 1995; 52:1333- 1341.
Scientific approach to the assessment and management of activit-related spinal disorders. A monograph for clinicians. Report of the Quebec Task Force on spinal disorders. Spine 1987; 12(Suppl 7):1-59.
Videman T, Saina S, Crites Battle M, Koskinen S, Gill K, Paanaman H. The long term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine 1995; 20:699-709.
Anderson GBJ, McNeill TW. Lumbar Spine Syndromes Evaluation and Treatment. New York: Springer-Veriag Wien, 1989: pp.1-215.
Olmarker K, Rydeuik B, Holm S, et al. Effects of experimental graded compression on blood-flow in spinal nerve roots. J Orthop Res 1989; 7:817-823.
Twomey LT. Sustained lumbar traction: An experimental study of long spine segments. Spine 1985; 10:146-149.
Gupta RC, Romarao SV. Epidurography in reduction of lumbar disc prolapse by traction. Arch Phys Med Rehabilitation 1978; 59:322-327.
Mathews JA. Dynamic discography: A study of lumbar traction. Ann Phys Med 1968; IV:275-279.
Judovich BC. Lumbar traction therapy-elimination of physical factors that prevent lumbar stretch. JAMA 1955; 159:549-550.
Ramos G, Martin W. Effects of vertebral axial decompression on intradiscal pressure. J Neurosurg 1994; 81:350-353.
Nachemson AL. The lumbar spine: An orthopaedic challenge. Spine 1975; 1:59-71.
Ballard WT, Weinstein JN. Biochemistry of the intervertebral disc. In: Kirkaldy-Willis WH, Burton CV, eds. Managing Low Back Pain, New York: Churchill Livingston, 1992: pp.39-48.
Gose EE, Johnsonbaugh R, Jost S. Pattern Recognition and Image Analysis, Upper Saddle River, NJ: Prentice- Hall PTR, 1996: pp.1-484.
